

Team up with a global leader in contrast imaging and solutions.
How to Order
LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use
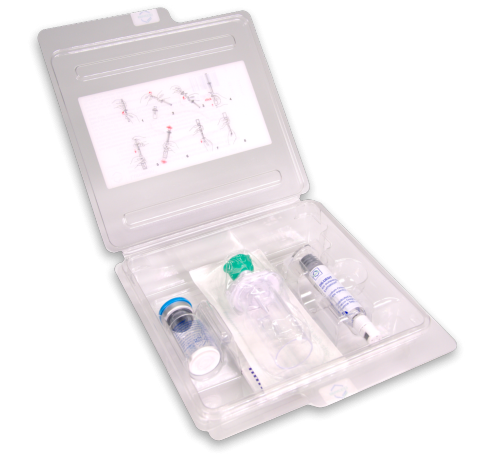
All-Inclusive Portable Kit
Each kit contains:
- One LUMASON vial of 25 mg lipid-type A white lyophilized
powder with headspace fill of 60.7 mg of sulfur hexafluoride - One prefilled syringe containing 5mL of 0.9% Sodium Chloride
Injection, USP (Diluent) - One Mini-Spike
| Product | LUMASON UEA |
|---|---|
| SKU | 709916 |
| NDC | 0270-7099-16 |
| Product Description | KIT LUMASON 5x 25MG FLIPCAP |
| Product Configuration | Five (5) Vial Kit |
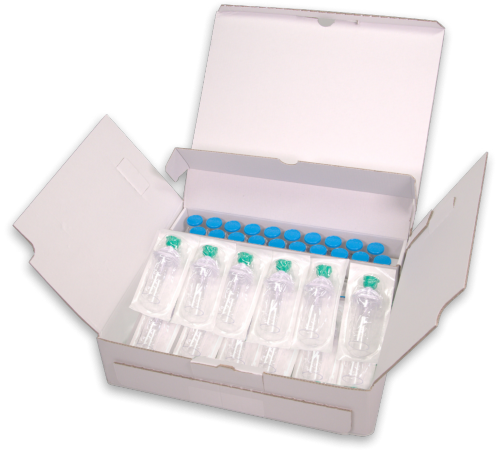
20-Vial Pack
Each pack contains:
- Twenty (20) LUMASON vials of 25 mg lipid-type A white lyophilized powder with headspace fill of 60.7 mg of sulfur hexafluoride
- Twenty (20) Mini-Spikes
- Twenty (20) peel-off syringe labels
| Product | LUMASON UEA |
|---|---|
| SKU | 709707 |
| NDC | 0270–7097–07 |
| Product Description | MP LUMASON 20x 25MG VIAL/SPIKE US |
| Product Configuration | Twenty (20) Vial Pack |
Available to purchase direct from Bracco Diagnostics Inc. and through authorized wholesale distributors.
Novaplus is a registered trademark of Vizient, Inc.

To order, call Bracco:
1-877-BRACCO-9
(1-877-272-2269)
or visit us online at:
MyOrders.Bracco.com
Reimbursement Support Information
Your one-stop resource for coding, reimbursement support, and education.
HCPCS Code
- LUMASON ultrasound enhancing agent (UEA) should be coded under Q9950: Inj sulf hexa lipid microsph.
CMS Hospital Outpatient Prospective Payment System
- Under HOPPS, reimbursement for LUMASON UEA is bundled in with the primary procedure and paid under the relevant Ambulatory Payment Classification.
- Please continue to include Q9950 on your charge master to assist CMS with future rate setting.
Medicare Physician Fee Schedule
- The MPFS is used in non-hospital sites including independent diagnostic testing facilities and physician offices.
- Under MPFS, LUMASON UEA is separately reimbursable from the primary procedure. Reimbursement rates change on a quarterly basis and are included in the CMS ASP Pricing files.
- Contrast agents are subject to the JW and JZ Modifiers under MPFS. Please use the JW Modifier to identify waste, and the JZ Modifier to indicate when there has been no waste.
Commercial Payors
- Commercial payors' policies and contracts can vary greatly across the country. Please check your individual agreements to find out how reimbursement for LUMASON UEA may impact your site.
Download the LUMASON Reimbursement Kit
Visit the Bracco reimbursement site
Disclaimers
The information provided is general reimbursement information for Bracco products. It is not legal advice, nor is it advice about how to code, complete or submit any particular claim for payment. Although we supply this information based on our current knowledge, it is always the provider’s responsibility to determine and submit appropriate codes, charges, modifiers and bills for services that were rendered. This coding and reimbursement information is subject to change without notice. Payers or their local branches may have their own coding and reimbursement requirements and policies. Before filing any claims, providers should verify current requirements and policies with the payer.
CPT® copyright 2024 American Medical Association (AMA). All rights reserved. Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein.
CPT is a registered trademark of the American Medical Association.
![]()
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use
Indications
LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use is an ultrasound contrast agent indicated for use:
- in echocardiography to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border in adult and pediatric patients with suboptimal echocardiograms
- in ultrasonography of the liver for characterization of focal liver lesions in adult and pediatric patients
- in ultrasonography of the urinary tract for the evaluation of suspected or known vesicoureteral reflux in pediatric patients
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
- Assess all patients for the presence of any condition that precludes administration
- Always have resuscitation equipment and trained personnel readily available
Contraindications
LUMASON (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use is contraindicated in patients with known or suspected hypersensitivity to sulfur hexafluoride lipid microsphere or its components, such as polyethylene glycol (PEG).
Warnings
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or shortly following administration of ultrasound contrast agents, including LUMASON. Always have cardiopulmonary resuscitation personnel and equipment readily available prior to LUMASON administration and monitor all patients for acute reactions.
Post-marketing hypersensitivity reactions, including serious hypersensitivity reactions, have been observed during use or shortly following LUMASON administration. These reactions may occur in patients with no history of prior exposure to sulfur hexafluoride lipid-containing microspheres. LUMASON contains PEG. There may be increased risk of serious reactions including death in patients with prior hypersensitivity reaction(s) to PEG.
Systemic embolization may occur in patients with cardiac shunts. Assess patients with cardiac shunts for embolic phenomena following LUMASON administration.
There is a risk of ventricular arrhythmia related to high mechanical index in patients administered LUMASON. LUMASON is not recommended for use at mechanical indices greater than 0.8.
The most common adverse reactions (incidence ≥ 0.5%) are headache (1%) and nausea (0.5%).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for LUMASON ultrasound contrast agent, including BOXED WARNING on Serious Cardiopulmonary Reactions.
LUMASON is manufactured for Bracco Diagnostics Inc., Princeton, NJ 08540 by Bracco Suisse S.A., Plan-les-Ouates Geneve, Switzerland (LUMASON lyophilized powder vial-25 mg lipid-type A/60.7 sulfur hexafluoride gas); Vetter Pharma-Fertigung GmbH & Co. KG, 88212 Ravensburg, Germany (Sodium Chloride 0.9% Injection, USP) or Bracco Imaging S.p.A. Via Ribes, 5, 10010 Colleretto Giacosa (TO), Italy (0.9% Sodium Chloride Injection, USP); B. Braun Melsungen AG 34212 Melsungen, Germany (Mini-Spike).
LUMASON and SONOVUE are registered trademarks of Bracco Diagnostics Inc. and its affiliated entities.
All other trademarks and registered trademarks are the property of their respective owners.

