
The individuals who appear are for illustrative purposes. All persons depicted are models and not real patients or healthcare professionals.
Only LUMASON® ultrasound enhancing agent has multiple indications for adult and pediatric patients.
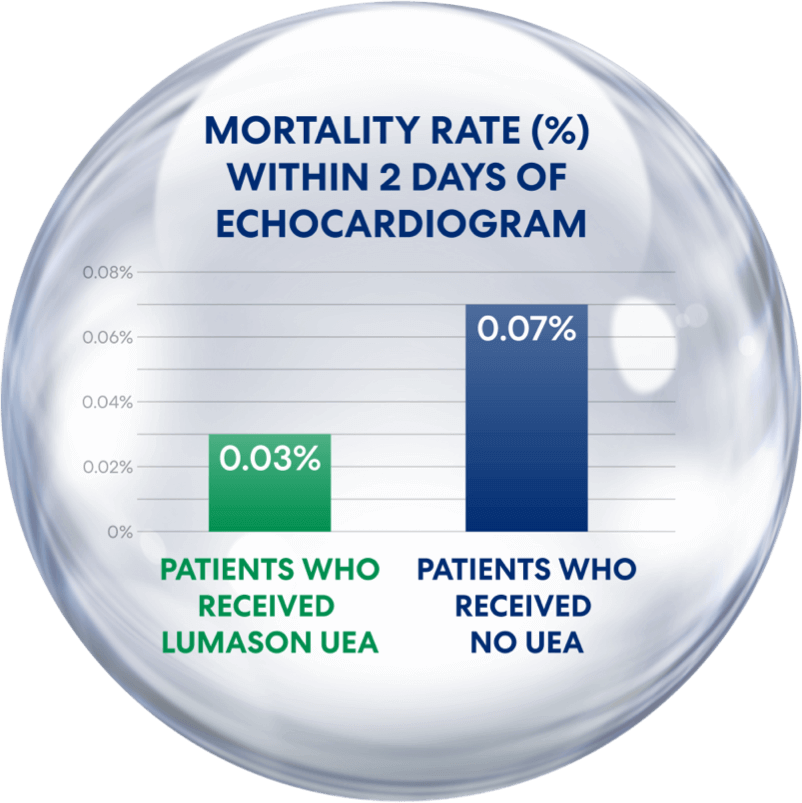
Compared to Non-Receipt of a UEA,
Receipt of LUMASON® UEA Was Associated With Reduced Mortality¹
Patients who received LUMASON UEA had a mortality rate of only 0.03%—lower than in those who didn’t receive a UEA
- Among patients who received UEAs, including LUMASON UEA, serious adverse event (SAE) rates were low and consistent with historical safety data (approximately 1 in 10,000)¹
- Given the low incidence of SAEs and association with reduced mortality, the study supports the safety of LUMASON UEA in cardiac imaging¹
Study by Strom et al., 2025¹
- The study “Contemporary Safety of Ultrasound Enhancing Agents in a Nationwide Analysis” was published in the Journal of American Heart Association. It utilized an extensive dataset from 11.4 million insured individuals across the United States from 2018–2022.
- This comprehensive analysis aimed to evaluate the rates of death, anaphylaxis, myocardial infarction, ventricular tachycardia, or cardiac arrest within 2 days of receiving—or not receiving—a UEA.
- Specifically, it found:
- SAEs associated with UEAs were uncommon and similar across types of UEA including LUMASON and years of study1
- People who received UEAs had lower odds of death within 2 days of receiving an echocardiogram1
- SAE rates were consistent across both pre-COVID and COVID-19 years1
- These findings not only reaffirm the safety of UEAs like LUMASON, but also suggest that their use may reduce the risk of death and help further our understanding of the safety and potential benefits of UEAs, helping to position them as reliable tools for enhancing diagnostic accuracy.1
- It is important to remember that despite the recent conversation around UEAs and SAEs, the overall reporting rate of SAEs to LUMASON UEA is low (~1 in 10,000 exposures) and appears to be similar to that reported for other ultrasound enhancing agents.1,2
Read the full study, “Contemporary Safety of Ultrasound Enhancing Agents in a Nationwide Analysis,” and/or contact your Bracco sales representative to discuss this new information.
Another study—Fillipone et al.—analyzed reported data of mortality rates among critically ill patients who received LUMASON UEA
The Fillipone study reported that echocardiography patients who were administered LUMASON UEA experienced a reduction in mortality compared with patients who were not administered a UEA.
Study by Filippone et al., 2023²
The paper by Filippone et al. reported data from prospective and retrospective clinical studies in critically ill patients at higher than usual risk for developing cardiopulmonary reactions, as well as post-marketing surveillance data up to February 2023.
- No detrimental effects of LUMASON UEA on cardiac electrophysiology were observed in patients with coronary artery disease, and no significant effects on pulmonary hemodynamics were noted in patients with pulmonary hypertension or congestive heart failure.
- Similarly, no effects on several assessments of pulmonary function were observed in patients with chronic obstructive pulmonary disease, and no clinically meaningful changes in oxygen saturation or other safety parameters were observed after administration of LUMASON UEA to patients with diffuse interstitial pulmonary fibrosis.
- The retrospective study of critically ill patients revealed a reduction of in-hospital mortality between patients administered LUMASON UEA for echocardiography versus those who had undergone echocardiography without a contrast agent.
- Post-marketing surveillance revealed very low reporting rates for all serious adverse events.
Read the study “Safety of LUMASON® (SonoVue®) in Special Populations and Critically Ill Patients”
As a company dedicated to advancing the field of contrast-enhanced ultrasound (CEUS), Bracco takes any observations about the safe use of products like LUMASON UEA seriously. Our priority is to ensure that every Bracco product is safe and effective for patients and of the highest quality.
To support this commitment, Bracco maintains a robust, global system to constantly monitor the quality and safety of its products, collaborate with health authorities, and regularly provide integrated medical safety evaluations and benefit-risk assessments.
Read the study “Safety Profile of Ultrasound Enhancing Agents in Echocardiography”

The individual who appears is for illustrative purposes. The person depicted is a model and not a real healthcare professional.
![]()
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use
Indications
LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use is an ultrasound contrast agent indicated for use:
- in echocardiography to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border in adult and pediatric patients with suboptimal echocardiograms
- in ultrasonography of the liver for characterization of focal liver lesions in adult and pediatric patients
- in ultrasonography of the urinary tract for the evaluation of suspected or known vesicoureteral reflux in pediatric patients
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
- Assess all patients for the presence of any condition that precludes administration
- Always have resuscitation equipment and trained personnel readily available
Contraindications
LUMASON (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use is contraindicated in patients with known or suspected hypersensitivity to sulfur hexafluoride lipid microsphere or its components, such as polyethylene glycol (PEG).
Warnings
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or shortly following administration of ultrasound contrast agents, including LUMASON. Always have cardiopulmonary resuscitation personnel and equipment readily available prior to LUMASON administration and monitor all patients for acute reactions.
Post-marketing hypersensitivity reactions, including serious hypersensitivity reactions, have been observed during use or shortly following LUMASON administration. These reactions may occur in patients with no history of prior exposure to sulfur hexafluoride lipid-containing microspheres. LUMASON contains PEG. There may be increased risk of serious reactions including death in patients with prior hypersensitivity reaction(s) to PEG.
Systemic embolization may occur in patients with cardiac shunts. Assess patients with cardiac shunts for embolic phenomena following LUMASON administration.
There is a risk of ventricular arrhythmia related to high mechanical index in patients administered LUMASON. LUMASON is not recommended for use at mechanical indices greater than 0.8.
The most common adverse reactions (incidence ≥ 0.5%) are headache (1%) and nausea (0.5%).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for LUMASON ultrasound contrast agent, including BOXED WARNING on Serious Cardiopulmonary Reactions.
LUMASON is manufactured for Bracco Diagnostics Inc., Princeton, NJ 08540 by Bracco Suisse S.A., Plan-les-Ouates Geneve, Switzerland (LUMASON lyophilized powder vial-25 mg lipid-type A/60.7 sulfur hexafluoride gas); Vetter Pharma-Fertigung GmbH & Co. KG, 88212 Ravensburg, Germany (Sodium Chloride 0.9% Injection, USP) or Bracco Imaging S.p.A. Via Ribes, 5, 10010 Colleretto Giacosa (TO), Italy (0.9% Sodium Chloride Injection, USP); B. Braun Melsungen AG 34212 Melsungen, Germany (Mini-Spike).
LUMASON and SONOVUE are registered trademarks of Bracco Diagnostics Inc. and its affiliated entities.
All other trademarks and registered trademarks are the property of their respective owners.
IMPORTANT SAFETY INFORMATION | INDICATION AND USAGE
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
References:
1. Strom JB, Mulvagh SL, Porter TR, et al. Contemporary safety of ultrasound enhancing agents in a nationwide analysis. J Am Heart Assoc. 2025;14:e039480. DOI: 10.1161/JAHA.124.039480
2. Filippone A, Kirchin MA, Monteith J, et al. Safety of LUMASON® (SonoVue®) in special populations and critically ill patients. Front Cardiovasc Med. 2023 Aug 2;10:1225654. doi: 10.3389/ fcvm.2023.1225654. PMID: 37600063; PMCID: PMC10433219.
3. Data on file. Bracco Diagnostics Inc.; June 2023

