Every Bubble
Tells a Story.
The individuals who appear are for illustrative purposes only. All persons depicted are models and not real patients.
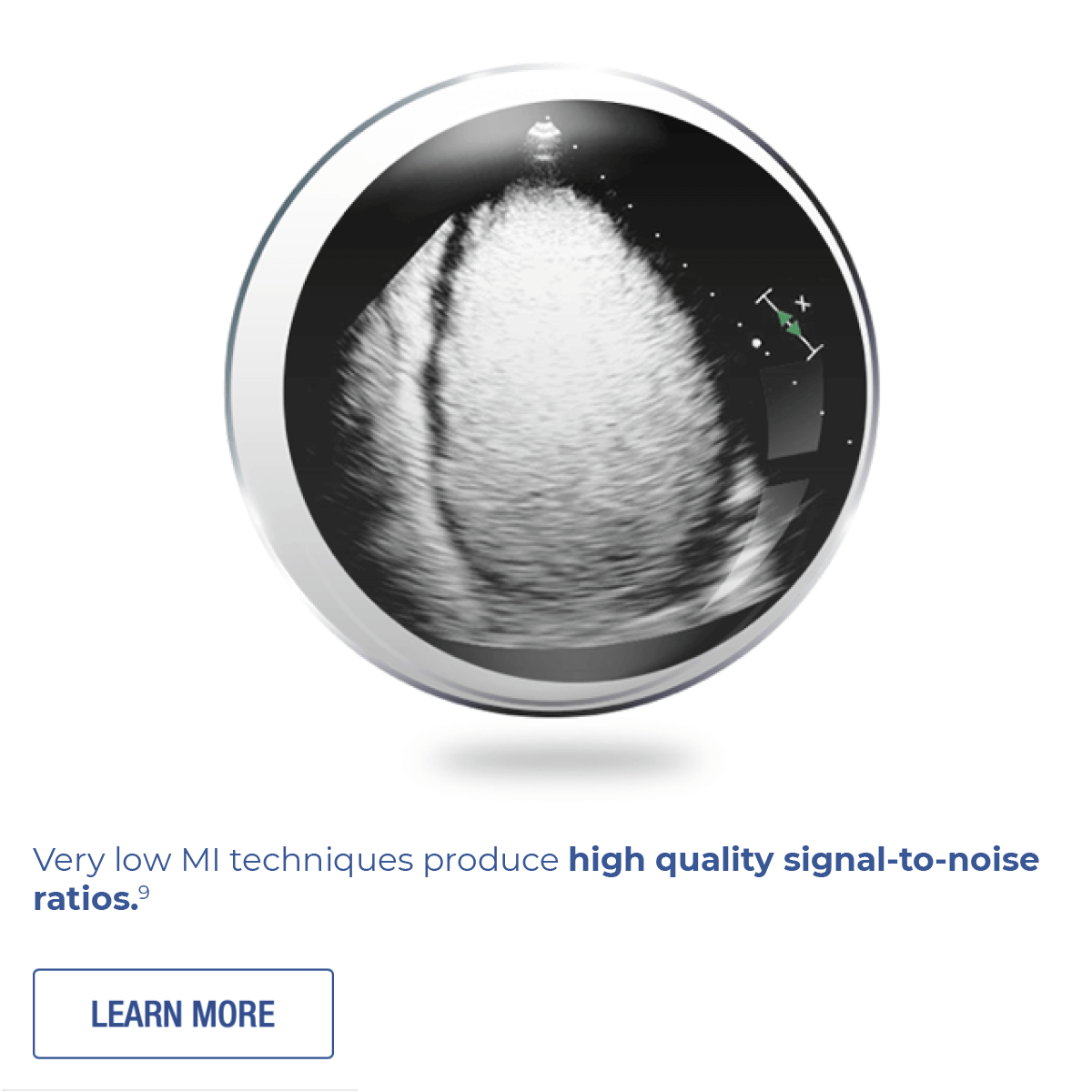
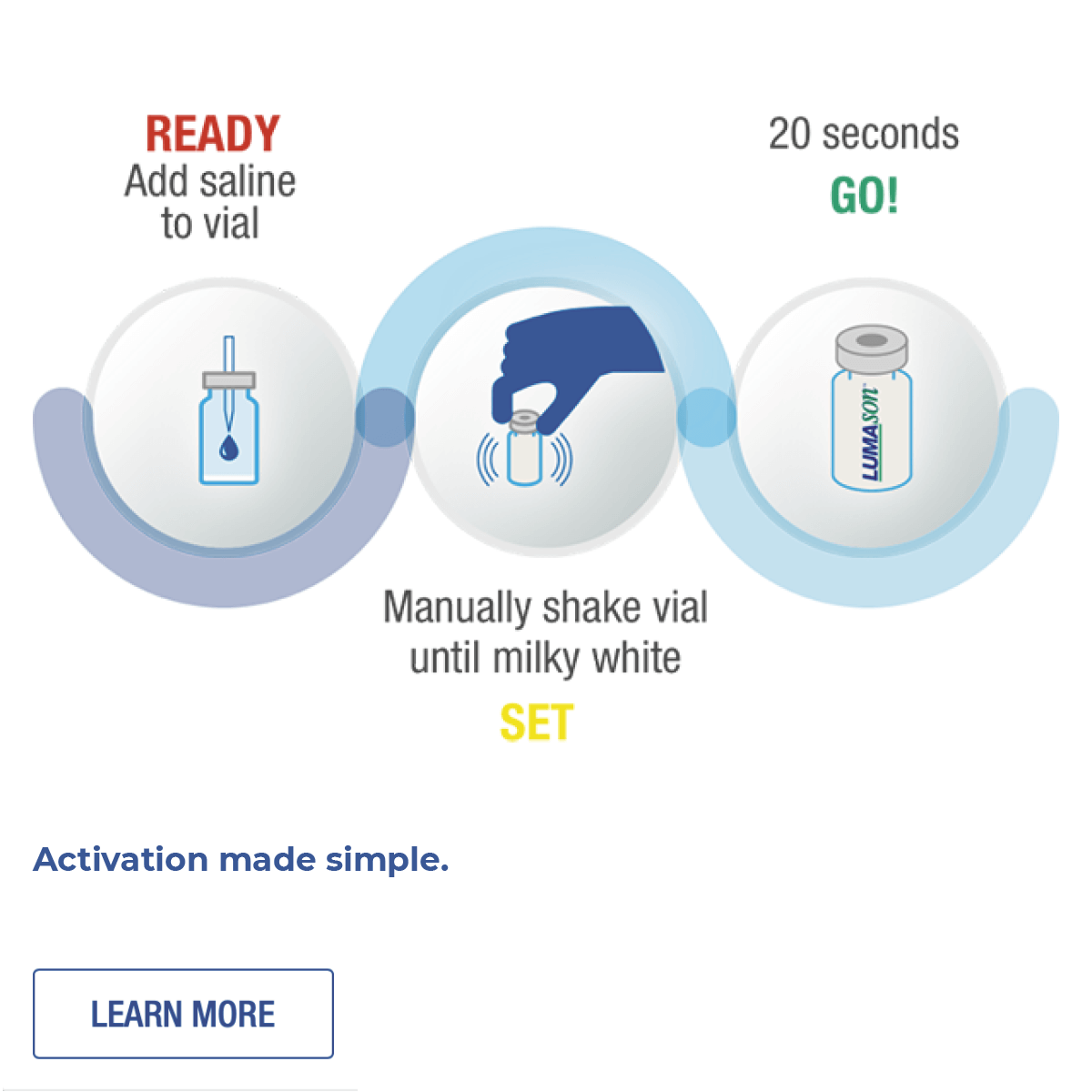
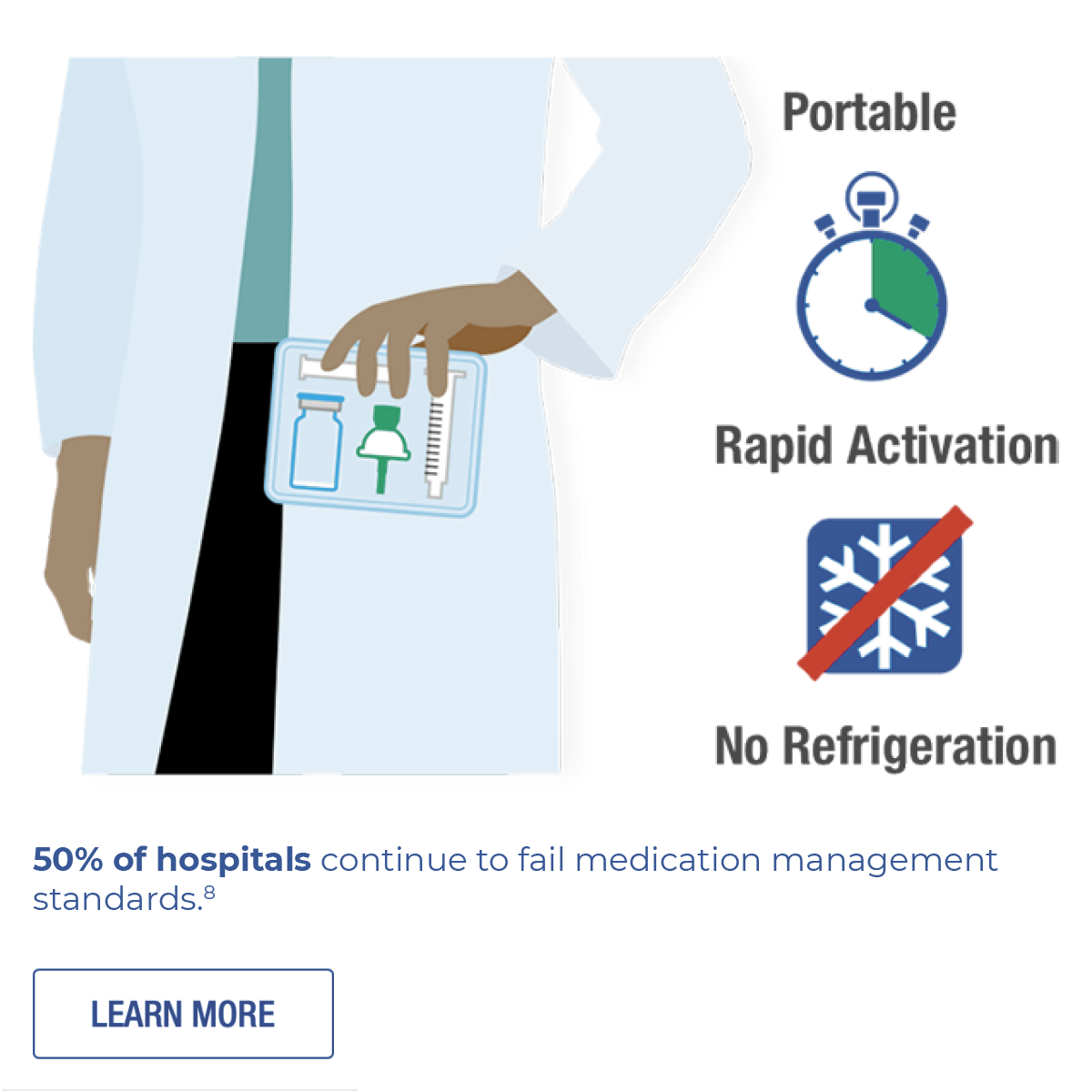
The microspheres provide high-resolution imaging at very low MI, never need refrigeration, and manually activate in seconds.1,12
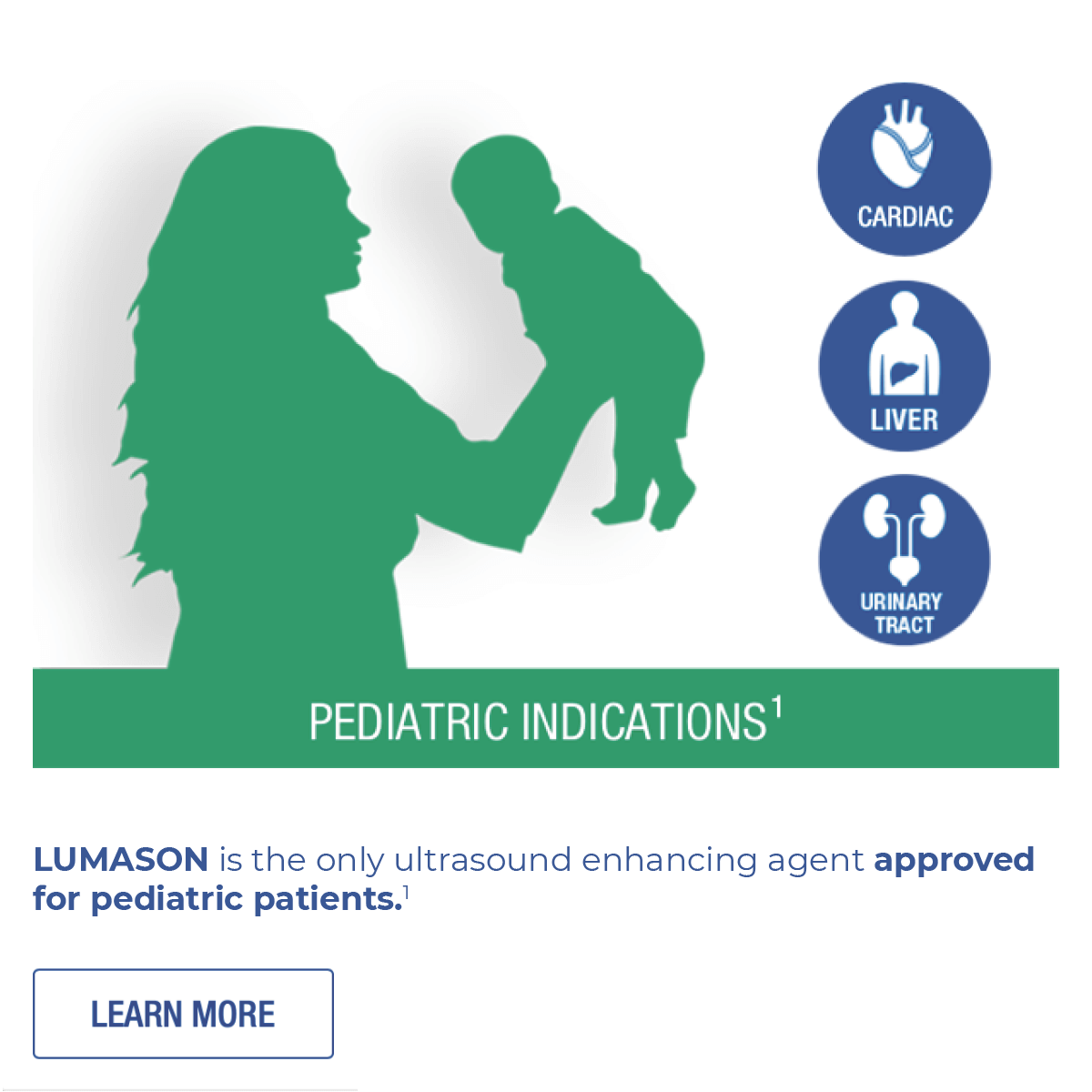
ONLY LUMASON ultrasound enhancing agent has multiple indications for adult and pediatric patients.
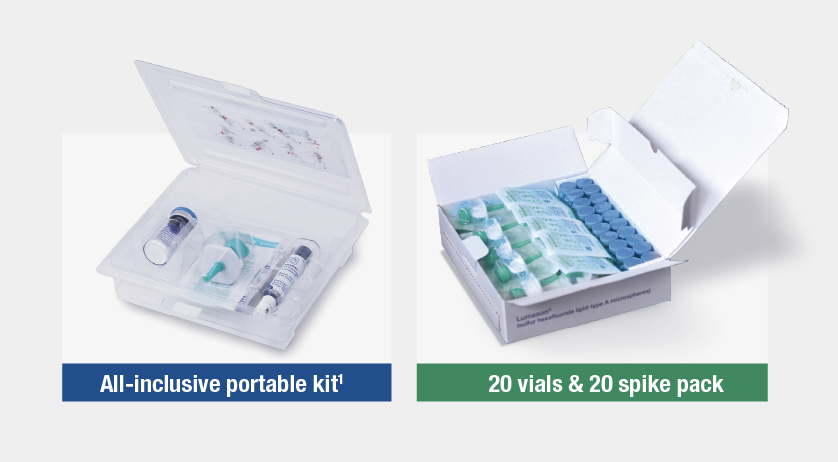
How Will Your Efficiency Be Enhanced With More Packaging Options
LUMASON® Ultrasound Enhancing Agent is available in two different packaging configurations, never needs refrigeration, and manually reconstitutes in seconds.1

Bracco Diagnostics is proud to offer LUMASON ultrasound enhancing agent as Novaplus®.
Vizient members, contact your Account Manager today
Novaplus is a registered trademark of Vizient, Inc. Vizient Contract XR0552
SKU 709700 : 5-Vial Kit
SKU 709708 : 20-Vial Pack

Bracco Diagnostics is proud to offer LUMASON ultrasound enhancing agent as Novaplus®.
Vizient members, contact your Account Manager today
SKU 709700 : 5-Vial Kit
SKU 709708 : 20-Vial Pack
Novaplus is a registered trademark of Vizient, Inc. Vizient Contract XR0552

Explore the
LUMASON Advantage
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use
Indications
LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use is an ultrasound contrast agent indicated for use:
- in echocardiography to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border in adult and pediatric patients with suboptimal echocardiograms
- in ultrasonography of the liver for characterization of focal liver lesions in adult and pediatric patients
- in ultrasonography of the urinary tract for the evaluation of suspected or known vesicoureteral reflux in pediatric patients
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
- Assess all patients for the presence of any condition that precludes administration
- Always have resuscitation equipment and trained personnel readily available
Contraindications
LUMASON (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use is contraindicated in patients with known or suspected hypersensitivity to sulfur hexafluoride lipid microsphere or its components, such as polyethylene glycol (PEG).
Warnings
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or shortly following administration of ultrasound contrast agents, including LUMASON. Always have cardiopulmonary resuscitation personnel and equipment readily available prior to LUMASON administration and monitor all patients for acute reactions.
Post-marketing hypersensitivity reactions, including serious hypersensitivity reactions, have been observed during use or shortly following LUMASON administration. These reactions may occur in patients with no history of prior exposure to sulfur hexafluoride lipid-containing microspheres. LUMASON contains PEG. There may be increased risk of serious reactions including death in patients with prior hypersensitivity reaction(s) to PEG.
Systemic embolization may occur in patients with cardiac shunts. Assess patients with cardiac shunts for embolic phenomena following LUMASON administration.
There is a risk of ventricular arrhythmia related to high mechanical index in patients administered LUMASON. LUMASON is not recommended for use at mechanical indices greater than 0.8.
The most common adverse reactions (incidence ≥ 0.5%) are headache (1%) and nausea (0.5%).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for LUMASON ultrasound contrast agent, including BOXED WARNING on Serious Cardiopulmonary Reactions.
LUMASON is manufactured for Bracco Diagnostics Inc., Monroe Township, NJ 08831 by Bracco Suisse S.A., Plan-les-Ouates Geneve, Switzerland (LUMASON lyophilized powder vial-25 mg lipid-type A/60.7 sulfur hexafluoride gas); Vetter Pharma-Fertigung GmbH & Co. KG, 88212 Ravensburg, Germany (Sodium Chloride 0.9% Injection, USP); B. Braun Melsungen AG, 34212 Melsungen, Germany (Mini-Spike).
LUMASON and SONOVUE are registered trademarks of Bracco Diagnostics Inc. and its affiliated entities.
All other trademarks and registered trademarks are the property of their respective owners.
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
References
- LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use full Prescribing Information. Monroe Twp., NJ: Bracco Diagnostics Inc.; August 2021.
- Papadopoulou F, Ntoulia A, Siomou E, Darge K. Contrast-enhanced voiding urosonography with intravesical administration of a second-generation ultrasound contrast agent for diagnosis of vesicoureteral reflux: prospective evaluation of contrast safety in 1,010 children. Pediatr Radiol. 2014;44(6):719-728.
- Trillaud H, Bruel JM, Valette PJ, et al. Characterization of focal liver lesions with SonoVue-enhanced sonography: International multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15(30):3748-3756.
- Schneider M. Characteristics of SonoVue™. Echocardiography. 1999;16(7, pt 2):743-746.
- Schneider M, Arditi M, Barrau MB, et al. BR1: a new ultrasound contrast agent based on sulfur hexafluoride-filled microbubbles. Invest Radiol. 1995;30(8):451-457.
- Data on file. Bracco Diagnostics Inc.
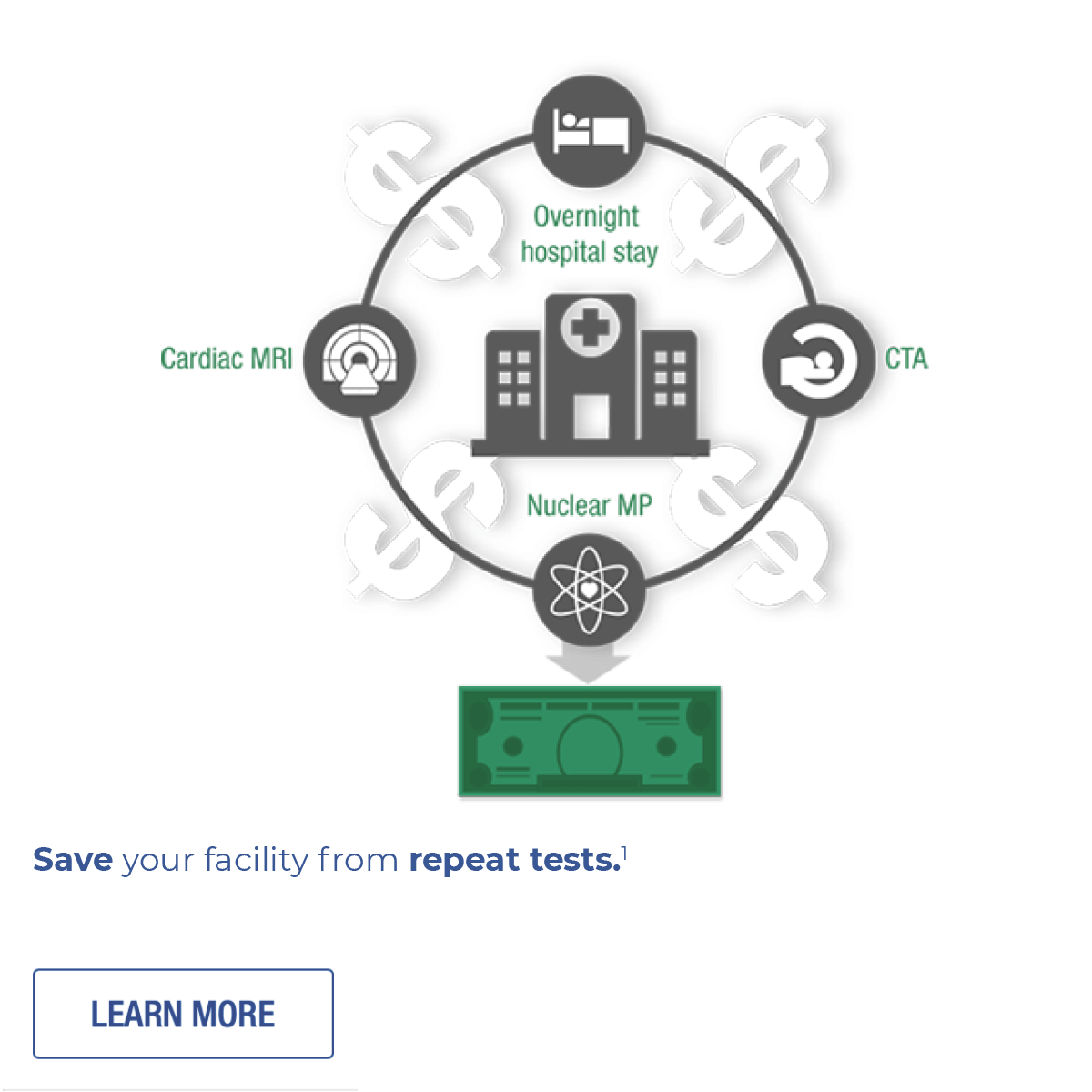
- Echocardiography procedures in Medicare Outpatient 2017 actual data update. Millennium Research Group. October 2018.
- Joyce MC. Your Annual Joint Commission Update. Presented at APhA2019 annual meeting and exposition. Seattle Washington, March 22-25, 2019. Data on file.
- Porter TR, Mulvagh SL, Abdelmoneim SS, et al, Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J AM Soc Echocardiogr. 2018;31(3):241-274.
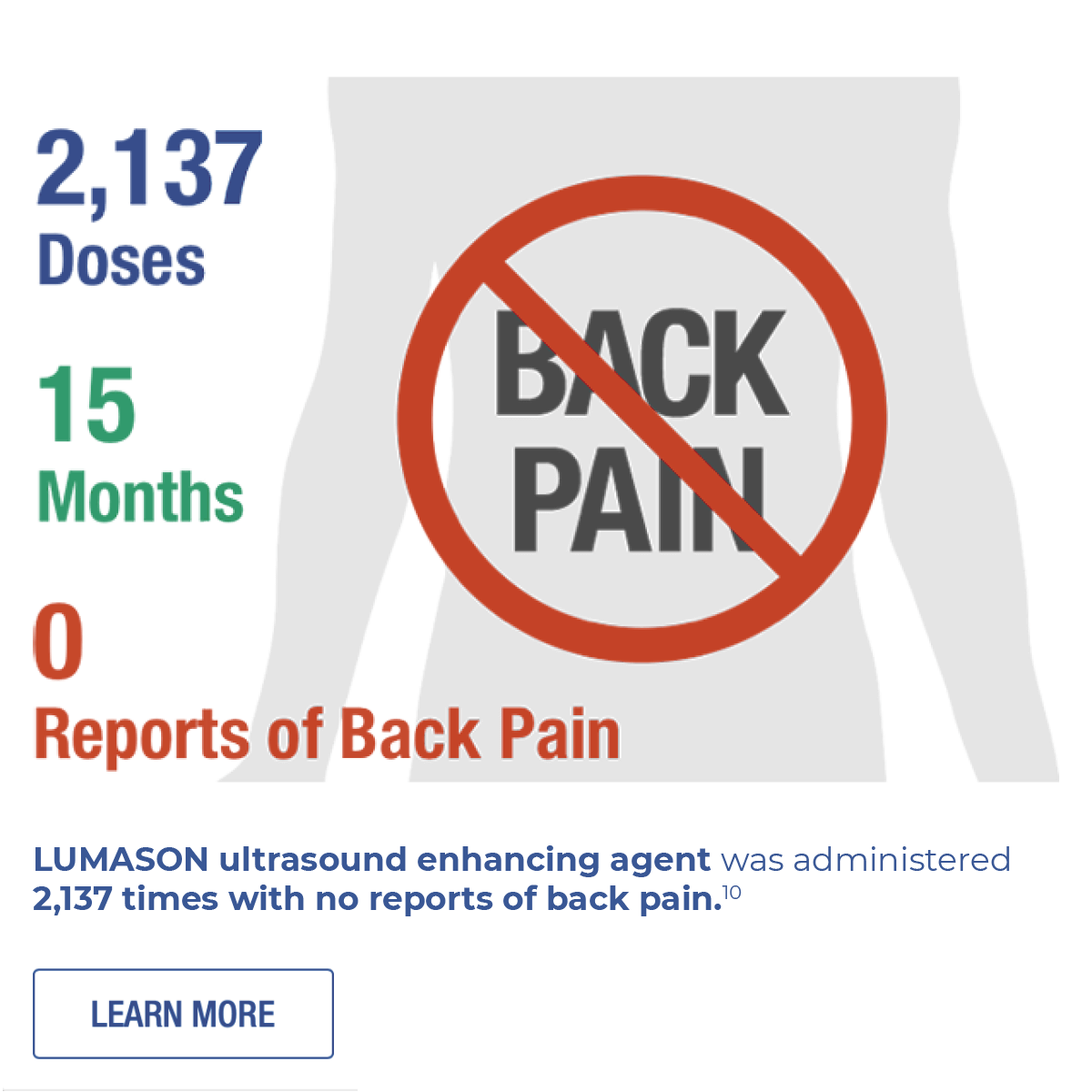
- Kumar S, Purtell C, Peterson A, Gibbons P, Khan AM, Heitner SB. Safety profile of ultrasound enhancing agents in echocardiography. Echocardiology. 2019;00:1–4:doi: https://doi.org/10.1111/echo.14344
- © 2020 Millennium Research Group, Inc. All rights reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission.
- Data on file. Bracco Diagnostics Inc. May 2019. Geneva data 2019.