Administer
Two different packaging configurations for easy, rapid reconstitution1
Watch the step-by-step video for reconstitution of LUMASON® UEA

All-inclusive portable kit
Each kit contains:
- One LUMASON vial of 25 mg lipid-type A white lyophilized powder with headspace fill of 60.7 mg of sulfur hexafluoride
- One prefilled syringe containing 5mL of 0.9% Sodium Chloride Injection, USP (Diluent)
- One Mini-Spike
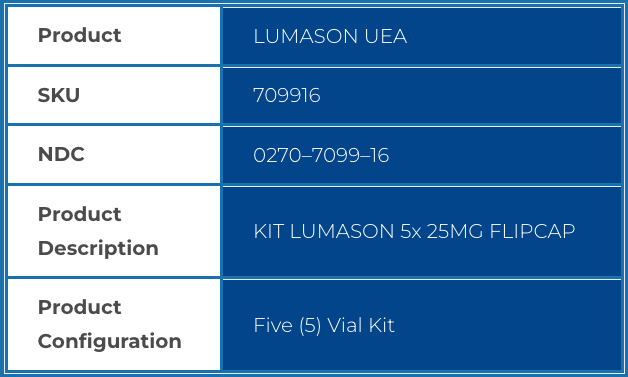
| Product | LUMASON UEA |
|---|---|
| SKU | 709916 |
| NDC | 0270–7099–16 |
| Product Description |
KIT LUMASON 5x 25MG FLIPCAP |
| Product Configuration |
Five (5) Vial Kit |
Novaplus® SKU 709700 NDC 0270-7099-73

20-vial pack
Each pack contains:
- Twenty (20) LUMASON vials of 25 mg lipid-type A white lyophilized powder with headspace fill of 60.7 mg of sulfur hexafluoride
- Twenty (20) Mini-Spikes
- Twenty (20) peel-off syringe labels
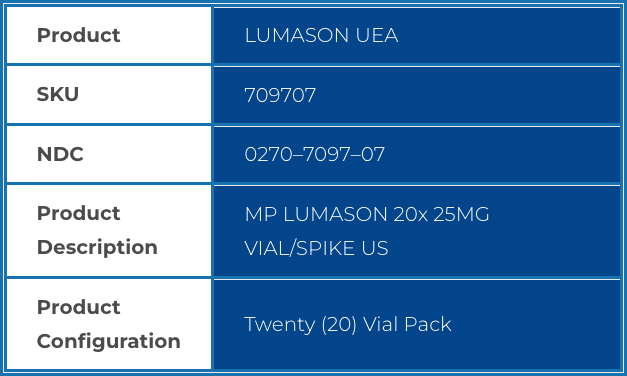
| Product | LUMASON UEA |
|---|---|
| SKU | 709707 |
| NDC | 0270–7097–07 |
| Product Description |
MP LUMASON 20x 25MG VIAL/SPIKE US |
| Product Configuration |
Twenty (20) Vial Pack |
Novaplus® SKU 709708 NDC 0270-7097-08
Available to purchase direct from Bracco Diagnostics Inc. and through authorized wholesale distributors.
Novaplus is a registered trademark of Vizient, Inc.
The LUMASON portable kit and 20-vial pack simplify administration.
- Agitate in hand
- No activation device required
- No refrigeration required
- Administration in 20 seconds

LUMASON does not require refrigeration

The individual who appears is for illustrative purposes. The person depicted is a model and not a real healthcare professional.
LUMASON Dosage and Administration1



*Follow LUMASON injection with an intravenous flush of 0.9% Sodium Chloride injection, USP.
†Do not exceed 2.0 mL per injection.
‡Do not exceed 2.4 mL per injection.
§The bladder may be refilled with normal saline for a second cycle of voiding and imaging, without the need of a second LUMASON administration.
Customer Service: 1-877-BRACCO 9 (1-877-272-2269)
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use
Indications
LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use is an ultrasound contrast agent indicated for use:
- in echocardiography to opacify the left ventricular chamber and to improve the delineation of the left ventricular endocardial border in adult and pediatric patients with suboptimal echocardiograms
- in ultrasonography of the liver for characterization of focal liver lesions in adult and pediatric patients
- in ultrasonography of the urinary tract for the evaluation of suspected or known vesicoureteral reflux in pediatric patients
IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.
- Assess all patients for the presence of any condition that precludes administration
- Always have resuscitation equipment and trained personnel readily available
Contraindications
LUMASON (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use is contraindicated in patients with known or suspected hypersensitivity to sulfur hexafluoride lipid microsphere or its components, such as polyethylene glycol (PEG).
Warnings
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or shortly following administration of ultrasound contrast agents, including LUMASON. Always have cardiopulmonary resuscitation personnel and equipment readily available prior to LUMASON administration and monitor all patients for acute reactions.
Post-marketing hypersensitivity reactions, including serious hypersensitivity reactions, have been observed during use or shortly following LUMASON administration. These reactions may occur in patients with no history of prior exposure to sulfur hexafluoride lipid-containing microspheres. LUMASON contains PEG. There may be increased risk of serious reactions including death in patients with prior hypersensitivity reaction(s) to PEG.
Systemic embolization may occur in patients with cardiac shunts. Assess patients with cardiac shunts for embolic phenomena following LUMASON administration.
There is a risk of ventricular arrhythmia related to high mechanical index in patients administered LUMASON. LUMASON is not recommended for use at mechanical indices greater than 0.8.
The most common adverse reactions (incidence ≥ 0.5%) are headache (1%) and nausea (0.5%).
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please click here for full Prescribing Information for LUMASON ultrasound contrast agent, including BOXED WARNING on Serious Cardiopulmonary Reactions.
LUMASON is manufactured for Bracco Diagnostics Inc., Monroe Township, NJ 08831 by Bracco Suisse S.A., Plan-les-Ouates Geneve, Switzerland (LUMASON lyophilized powder vial-25 mg lipid-type A/60.7 sulfur hexafluoride gas); Vetter Pharma-Fertigung GmbH & Co. KG, 88212 Ravensburg, Germany (Sodium Chloride 0.9% Injection, USP); B. Braun Melsungen AG, 34212 Melsungen, Germany (Mini-Spike).
LUMASON and SONOVUE are registered trademarks of Bracco Diagnostics Inc. and its affiliated entities.
All other trademarks and registered trademarks are the property of their respective owners.
References
- LUMASON® (sulfur hexafluoride lipid-type A microspheres) for injectable suspension, for intravenous use or intravesical use full Prescribing Information. Monroe Twp., NJ: Bracco Diagnostics Inc.; August 2021.
- Papadopoulou F, Ntoulia A, Siomou E, Darge K. Contrast-enhanced voiding urosonography with intravesical administration of a second-generation ultrasound contrast agent for diagnosis of vesicoureteral reflux: prospective evaluation of contrast safety in 1,010 children. Pediatr Radiol. 2014;44(6):719-728.
- Trillaud H, Bruel JM, Valette PJ, et al. Characterization of focal liver lesions with SonoVue-enhanced sonography: International multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15(30):3748-3756.
- Schneider M. Characteristics of SonoVue™. Echocardiography. 1999;16(7, pt 2):743-746.
- Schneider M, Arditi M, Barrau MB, et al. BR1: a new ultrasound contrast agent based on sulfur hexafluoride-filled microbubbles. Invest Radiol. 1995;30(8):451-457.
- Data on file. Bracco Diagnostics Inc.
- Echocardiography procedures in Medicare Outpatient 2017 actual data update. Millennium Research Group. October 2018.
- Joyce MC. Your Annual Joint Commission Update. Presented at APhA2019 annual meeting and exposition. Seattle Washington, March 22-25, 2019. Data on file.
- Porter TR, Mulvagh SL, Abdelmoneim SS, et al, Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J AM Soc Echocardiogr. 2018;31(3):241-274.
- Kumar S, Purtell C, Peterson A, Gibbons P, Khan AM, Heitner SB. Safety profile of ultrasound enhancing agents in echocardiography. Echocardiology. 2019;00:1–4:doi: https://doi.org/10.1111/echo.14344
- Data on file. Bracco Diagnostics Inc. May 2019. Geneva data 2019.
INDICATIONS AND USAGE | IMPORTANT SAFETY INFORMATION
WARNING: SERIOUS CARDIOPULMONARY REACTIONS
Serious cardiopulmonary reactions, including fatalities, have occurred uncommonly during or following the injection of ultrasound contrast agents, including sulfur hexafluoride lipid microspheres. Most serious reactions occur within 30 minutes of administration.


